Surgical Staples Litigation
The U.S. Food and Drug Administration recently issued a significant letter to health care providers about the safe use of surgical staplers and staples for internal use.
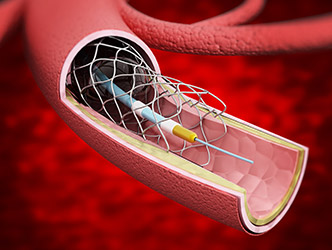
Arrow Endurance Peripheral Catheter
Arrow International and their parent company Teflex initiated the recall of the ARROW Endurance Extended Dwell Peripheral Catheter System due to reports of separation or leakage with the catheters.
DePuy Synthes Distraction System
In the interest of helping patients with mandibular defects, the DePuy Synthes Craniomaxillofacial Distraction System is an implant designed to lengthen and/or stabilize the lower jawbone.
Harbor Freight Jack Stand
Jack stands are used to safely support the weight of a vehicle while work is being performed, but if a jack stand fails injury or death can possibly occur to a person when they are defectively designed or defectively manufactured. Two different jack stand models sold by Harbor Freight have been recalled.
FiberCel Bone Graft Recall
On June 2, 2021, the U.S. Food & Drug Administration announced a voluntary product recall by regenerative medicine company Aziyo Biologics of their “FiberCel Viable Bone Matrix,” a fiber-based bone repair product made from human tissue.
MindFrameCapture LP Revascularization Devices
The FDA recently issued a Class 1 FDA recall (the most serious FDA recall), of several lots of MindFrameCapture LP Revascularization Devices due to a potentially lethal defect.
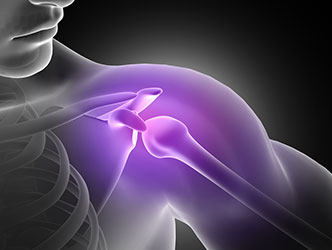
Modular Shoulder System Lawsuit
Some 50,000 shoulder replacement surgeries are done every year in the U.S. It is often recommended for those who are experiencing severe pain or stiffness due to arthritis or degenerative joint diseases.
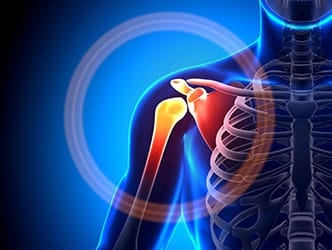
Zimmer Shoulder Recall
Zimmer Biomet recalled these devices because they were fracturing at a higher rate than the company had stated in its labeling.

Dehumidifier Lawsuits
Approximately 2.5 million units sold in the United States, and about 55,000 units sold in Canada, have been recalled.
Defective ARC Airbags
ARC Automotive Inc. is an international provider of airbag inflator technology facing lawsuit claims for defective inflators. These airbag inflators may explode and injure vehicle occupants and have been placed in over 30 million vehicles manufactured in the past decade.
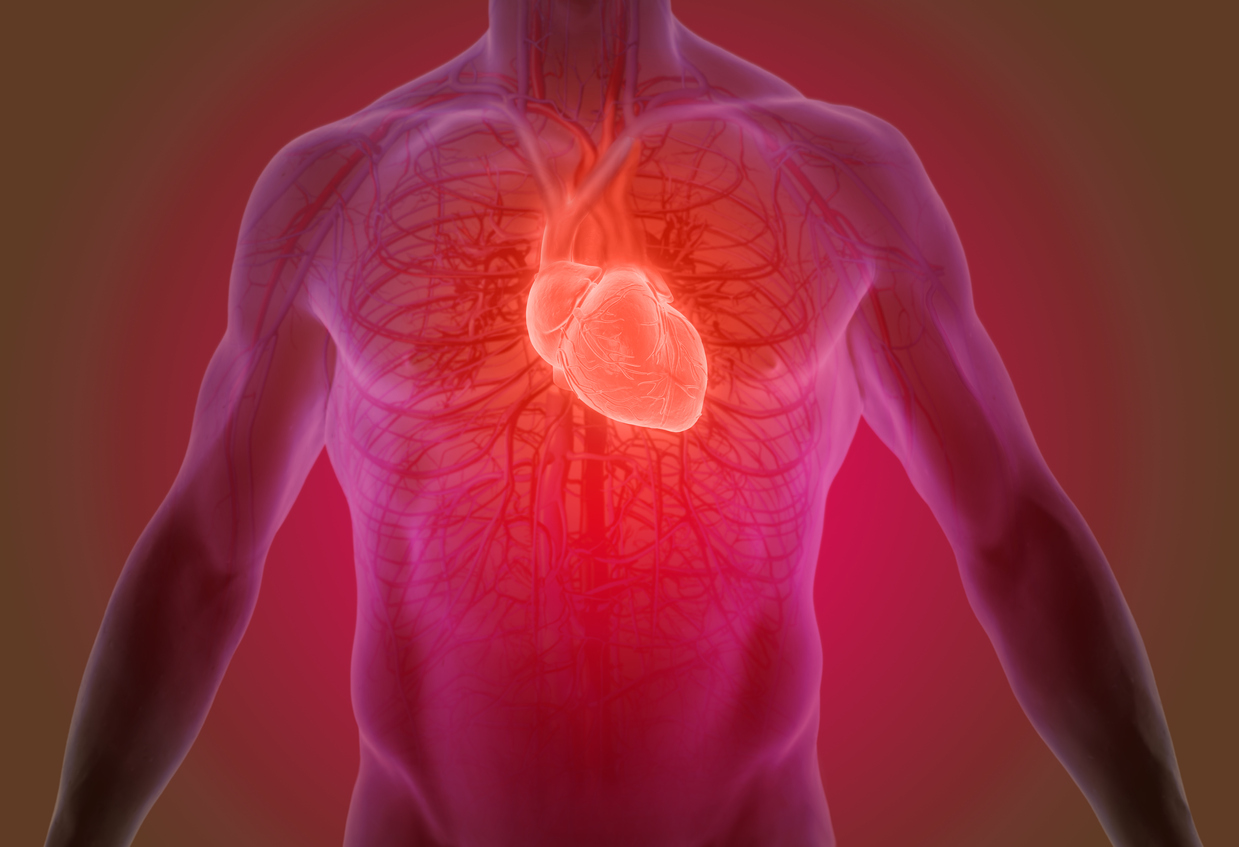
Abbott's Trifecta Heart Valve
In a recent development, Abbott, the manufacturer of the Trifecta heart valves, issued a voluntary recall.
MEGADYNE™ MEGA SOFT™ Reusable Patient Return Electrode
On July 11, 2023, the FDA labeled a Class I recall for Megadyne’s MEGA 2000 and MEGA electrodes products, manufactured by Johnson & Johnson’s Ethicon, due to the risk of serious burns to patients.